Cadmium sulfate
| |
| Names | |
|---|---|
| IUPAC name
Cadmium(II) sulfate
| |
| Other names
Sulfuric acid, cadmium salt (1:1),
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.288 |
| EC Number |
|
| 8295 | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2570 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CdSO4 CdSO4·H2O (monohydrate) 3CdSO4·8H2O (octahydrate) | |
| Molar mass | 208.47 g/mol (anhydrous) 226.490 g/mol (monohydrate) 769.546 g/mol (octahydrate) |
| Appearance | White hygroscopic solid |
| Odor | odorless |
| Density | 4.691 g/cm3 (anhydrous) 3.79 g/cm3 (monohydrate) 3.08 g/cm3 (octahydrate)[1] |
| Melting point | 1,000 °C (1,830 °F; 1,270 K) (anhydrous) 105 °C (monohydrate) 40 °C (octahydrate) |
| Boiling point | (decomposes to basic sulfate and then oxide) |
| anhydrous: 75 g/100 mL (0 °C) 76.4 g/100 mL (25 °C) 58.4 g/100 mL (99 °C) monohydrate: 76.7 g/100 mL (25 °C) octahydrate: very soluble | |
| Solubility | slightly soluble in methanol, ethyl acetate insoluble in ethanol |
| -59.2·10−6 cm3/mol | |
Refractive index (nD)
|
1.565 |
| Structure | |
| orthorhombic (anhydrous) monoclinic (hepta & octahydrate) | |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
123 J·mol−1·K−1[2] |
Std enthalpy of
formation (ΔfH⦵298) |
−935 kJ·mol−1[2] |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H301, H330, H340, H350, H360, H372, H410 | |
| P201, P202, P260, P264, P270, P271, P273, P281, P284, P301+P310, P304+P340, P308+P313, P310, P314, P320, P321, P330, P391, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
280 mg/kg (oral, rat) |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
[1910.1027] TWA 0.005 mg/m3 (as Cd)[3] |
REL (Recommended)
|
Ca[3] |
IDLH (Immediate danger)
|
Ca [9 mg/m3 (as Cd)][3] |
| Safety data sheet (SDS) | [1] |
| Related compounds | |
Other anions
|
Cadmium acetate, Cadmium chloride, Cadmium nitrate |
Other cations
|
Zinc sulfate, Calcium sulfate, Magnesium sulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cadmium sulfate is the name of a series of related inorganic compounds with the formula CdSO4·xH2O. The most common form is the monohydrate CdSO4·H2O, but two other forms are known CdSO4·8⁄3H2O and the anhydrous salt (CdSO4). All salts are colourless and highly soluble in water.
Structure, preparation, and occurrence
[edit]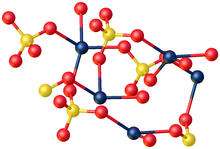
X-ray crystallography shows that CdSO4·H2O is a typical coordination polymer. Each Cd2+ center has octahedral coordination geometry, being surrounded by four oxygen centers provided by four sulfate ligands and two oxygen centers from the bridging water ligands.[5]
Cadmium sulfate hydrate can be prepared by the reaction of cadmium metal or its oxide or hydroxide with dilute sulfuric acid:
- CdO + H2SO4 → CdSO4 + H2O
- Cd + H2SO4 → CdSO4 + H2
The anhydrous material can be prepared using sodium persulfate:[citation needed]
- Cd + Na2S2O8 → CdSO4 + Na2SO4
Cadmium sulfates occur as the following rare minerals drobecite (CdSO4·4H2O), voudourisite (monohydrate), and lazaridisite (the 8/3-hydrate).
Applications
[edit]Cadmium sulfate is used widely for the electroplating of cadmium in electronic circuits. It is also a precursor to cadmium-based pigment such as cadmium sulfide. It is also used for electrolyte in a Weston standard cell as well as a pigment in fluorescent screens.
References
[edit]- ^ Lide, David R., ed. (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, Florida: CRC Press. ISBN 0-8493-0487-3.
- ^ a b Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A21. ISBN 978-0-618-94690-7.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0087". National Institute for Occupational Safety and Health (NIOSH).
- ^ Aurivillius, Karin; Stålhandske, Claes (1980). "A Reinvestigation of the Crystal Structures of HgSO4 and CdSO4". Zeitschrift für Kristallographie - Crystalline Materials. 153 (1–2): 121–129. Bibcode:1980ZK....153..121A. doi:10.1524/zkri.1980.0011.
- ^ Theppitak, C.; Chainok, K. (2015). "Crystal Structure of CdSO4(H2O): A Redetermination"". Acta Crystallographica Section E. 71 (10): i8 – pi9. doi:10.1107/S2056989015016904. PMC 4647421. PMID 26594423.

